

The 2010 version of the Oxford University Press style guide for authors in life sciences gave the following guidance " Use the Système international d'unités (SI) wherever possible.
#Atomic mass unit iso
In 2009, when the International Organization for Standardization published updated versions of ISO 80000, it gave mixed messages as to whether or not the unified atomic mass unit had been deprecated: ISO ISO 80000-1:2009 (General), identified the dalton as having " earlier called the unified atomic mass unit u", but ISO 80000-10:2009 (atomic and nuclear physics) catalogued both as being alternatives for each other.The definition also noted that " The dalton is often combined with SI prefixes. In 2006, in the 8th edition of the formal definition of SI, the CIPM cataloged the dalton alongside the unified atomic mass unit as a "Non-SI units whose values in SI units must be obtained experimentally: Units accepted for use with the SI".In 2005, the International Union of Pure and Applied Physics endorsed the use of the dalton as an alternative to the unified atomic mass unit.In 2003 the Consultative Committee for Units, part of the CIPM, recommended a preference for the usage of the " dalton" over the " unified atomic mass unit" as it " is shorter and works better with prefixes".

In 1993, the International Union of Pure and Applied Chemistry approved the use of the dalton with the qualification that the GCPM had not given its approval.With the introduction of the name "dalton", there has been a gradual change towards using that name in preference to the name "unified atomic mass unit".
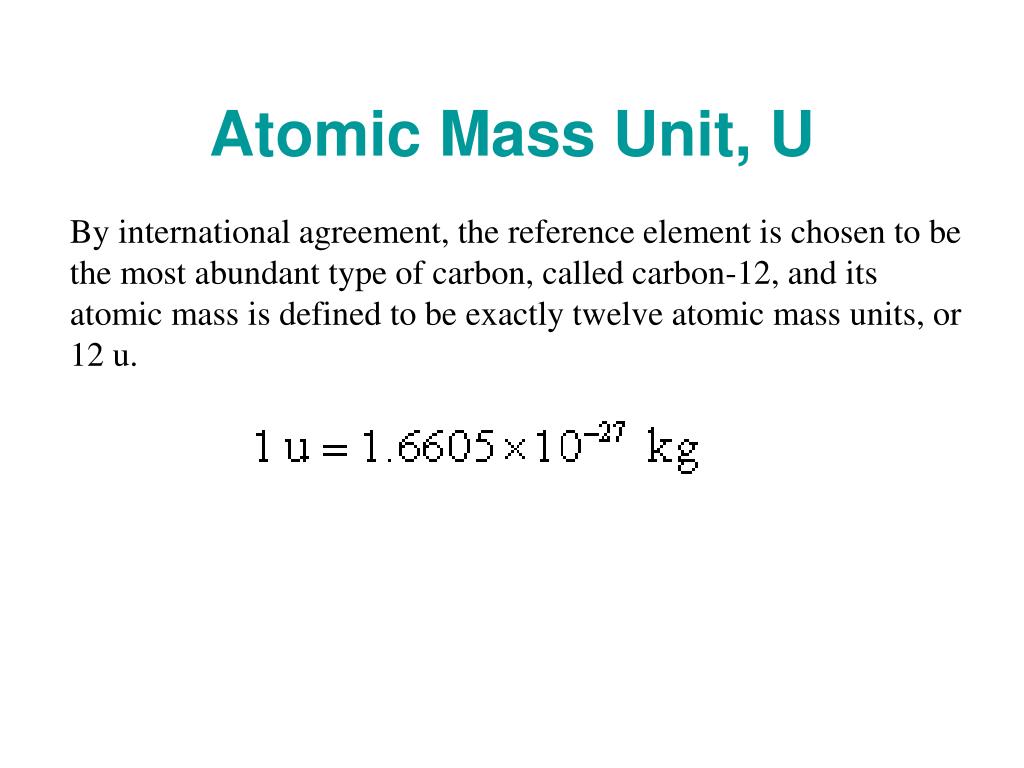
The unified atomic mass unit and the dalton are different names for the same unit of measure. The choice of carbon-12 was used to minimize further divergence with prior literature. The current unit is referred to as the "unified atomic mass unit" u. The reference was changed to carbon-12 in 1961 and a new symbol "u" replaced the now deprecated "amu". The inevitable divergence could result in errors in computations, and was thus unwieldy. The discovery of isotopic oxygen in 1929 led to a divergence in atomic weight representation, with isotopically weighted oxygen (chemistry) and pure 16O (physics) bases both used as the basis for the atomic mass unit (amu). This evaluation was made prior to the discovery of the existence of elemental isotopes, which occurred in 1912. Despite the initial mass of 1H being used as the natural unit for atomic weight, it was suggested by Wilhelm Ostwald that atomic weights would be best expressed in terms in units of 1/16 weight of oxygen. The atomic weight scale has traditionally been a relative scale, that is without an explicit unit, with the first atomic weight basis suggested by John Dalton in 1803 as 1H.


 0 kommentar(er)
0 kommentar(er)
